Immune checkpoint inhibitor
Drug discovery toward immune checkpoint LILRB4 and its prognostic application for cancer patients as a biomarker
Overview
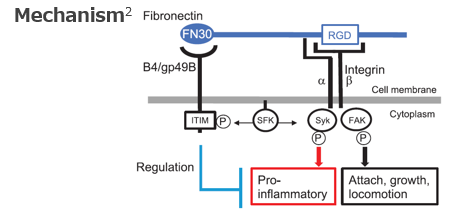
A myeloid checkpoint (CP), ILT3/LILRB4 (B4) is well-known and expected for cancer treatment, but its physiological ligand was unknown. This invention is about the physiological ligand of B4, fibronectin (FN), and its therapeutic use.
Following patterns can be considered for immunoregulation by blocking the binding between B4 and its ligand FN :
(1) FN analog (competitively binds to B4-FN)
(2) Anti-B4 antibody (acts on B4 and inhibits B4-FN binding) *
(3) Anti-FN antibody (acts on FN and inhibits B4-FN binding)
*Note: Anti-B4 antibody has been exclusively licensed to Company X, but small molecules and peptides that interact with B4, as well as (1) and (3) are free to develop.
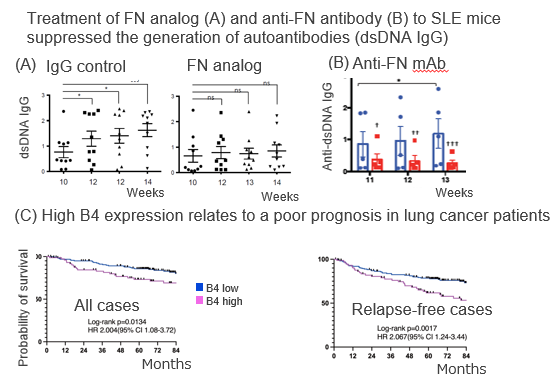
Further, B4 as a biomarker for lung cancer patients’ prognosis prediction was verified by our original B4 monoclonal antibody that inhibits B4-FN binding (Figure C, Patent ②).
Features・Outstandings
Product Application
・Drugs for treating autoimmune, cancer, inflammatory or allergic diseases associated with B4
・Diagnostic agent that predicts the effectiveness of cancer immunotherapy
・Diagnostic agents that predict cancer prognosis
Related Works
[1] International Immunology, 33(8), pp. 447–458.
[2] International Immunology, 34(8), pp. 435–444.
IP Data
IP No. : ①WO2021/029318, ②WO2023/002943
Inventor : TAKAI Toshiyuki, SU Mei Tzu, et al.
keyword : checkpoint, oncology, cancer prognosis, LILRB4, ILT3
