Recycling of Lithium-ion Battery Cathode Materials through Hydrothermal Reaction
Expected to reduce environmental load, avoid corrosion of equipment, and shorten reaction time
Overview
As a method for recovering valuable metals from spent LIB cathode materials, a wet scouring method is mainly used in which various metals are separated using a back extraction method using an organic solvent after leaching metal components using an acid. However, sulfuric acid and nitric acid, which are used as acids, cause a large environmental load because toxic gases are generated, and hydrogen peroxide, which is used as a reducing agent, has problems such as explosiveness and carcinogenicity. In a system without adding hydrogen peroxide, the recovery rate of metal ions decreases, so that improvement of the process is required.
In order to solve the above problems, the inventors focused on a hydrothermal reaction and examined reaction conditions such as the type of acid. As a result, they succeeded in almost completely leaching metals without using strong acids and reducing agents by using organic acids such as citric acid and the amino acid glycine. The effects of continuous operation, shortening of reaction time, and avoiding corrosion of equipment can be expected by the present invention.
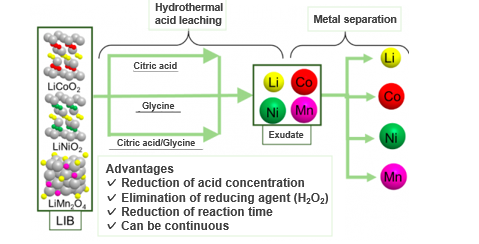
Excellent Performance of Glycine in Isolating metals during Hydrothermal Leaching[2]
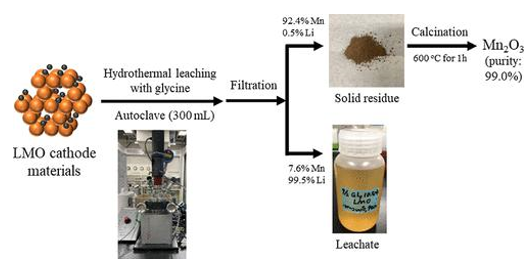
Product Application
・Recycling spent LIB cathode materials
・Metal resource recovery
・Mine development
Related Works
[1] JST Tohoku University New Technology Briefing, 2019
[2] ACS Sustainable Chemistry & Engineering, 11,35 (2023),13033–13042
IP Data
IP No. : JP7398080
Inventor : WATANABE Masaru, SHIBAZAKI Kensuke
keyword : rare metal, lithium ion battery, hydrothermal, reaching
